The coronavirus pandemic has changed life as we know it in just a few short months. Since the World Health Organisation declared it a Public Health Emergency of International Concern on 30 January, the search for a vaccine has involved thousands of researchers and volunteers around the world. One of the leading candidates is being developed in the UK by a group of Oxford University scientists.
Background
The Oxford COVID-19 vaccine team is led by Prof Sarah Gilbert, Prof Andrew Pollard, Prof Teresa Lambe, Dr Sandy Douglas, Prof Catherine Green and Prof Adrian Hill. Their team includes scientists from both the Jenner Institute and the Oxford Vaccine Group, who bring together decades of internationally recognised experience in vaccine research, including responding to the Ebola outbreak of 2014.
The teams had already used ChAdOx1 vaccine technology to produce candidate vaccines against a number of pathogens including flu, Zika and Middle East Respiratory Syndrome (MERS), another coronavirus. They had already begun work on pandemic preparedness with the technology behind ChAdOx, in preparation for 'Disease X'. When the disease emerged in China, they moved quickly. As soon as the genetic sequence was available, they began work on a trial.
How the Oxford COVID-19 vaccine works
The ChAdOx1 vaccine is a chimpanzee adenovirus vaccine vector. This is a harmless, weakened adenovirus that usually causes the common cold in chimpanzees. ChAdOx1 was chosen as the most suitable vaccine technology for a SARS-CoV-2 vaccine as it has been shown to generate a strong immune response from one dose in other vaccines. It has been genetically changed so that it is impossible for it to grow in humans. This also makes it safer to give to children, the elderly and anyone with a pre-existing condition such as diabetes. Chimpanzee adenoviral vectors are a very well-studied vaccine type, having been used safely in thousands of subjects.
Coronaviruses have club-shaped spikes on their outer coats, which form a corona – Latin for crown – on the virus surface. Immune responses from other coronavirus studies suggest that these spikes are a good target for a vaccine.
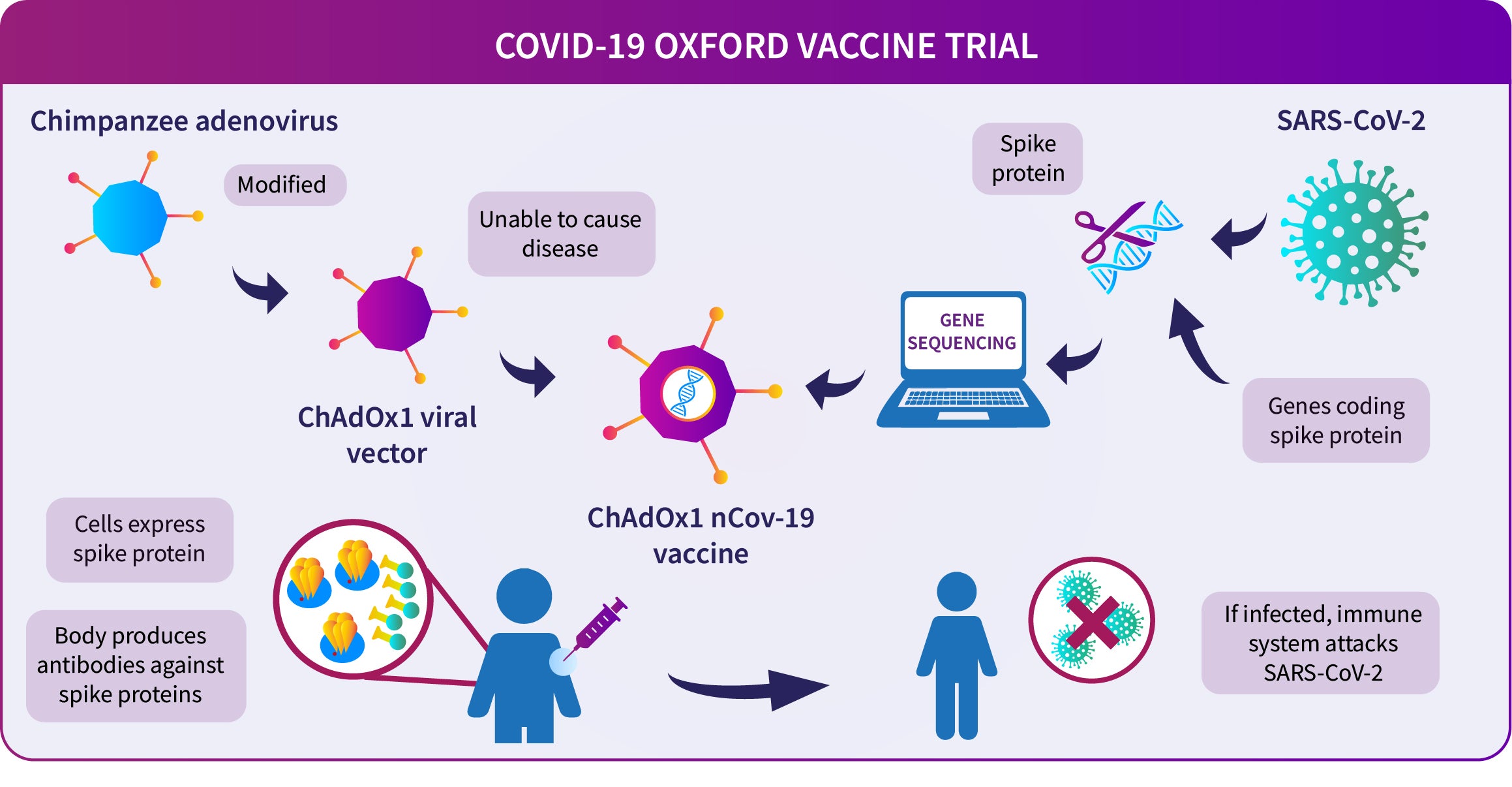 A diagram showing how the Oxford COVID-19 vaccine works. A chimpanzee adenovirus is used in the ChAdOx1 viral vector, engineered to match the SARS-CoV-2 spike protein.
A diagram showing how the Oxford COVID-19 vaccine works. A chimpanzee adenovirus is used in the ChAdOx1 viral vector, engineered to match the SARS-CoV-2 spike protein.A diagram showing how the Oxford COVID-19 vaccine works. A chimpanzee adenovirus is used in the ChAdOx1 viral vector, engineered to match the SARS-CoV-2 spike protein.
The Oxford vaccine contains the genetic sequence of this surface spike protein. When the vaccine enters cells inside the body, it uses this genetic code to produce the surface spike protein of the coronavirus. This induces an immune response, priming the immune system to attack the coronavirus if it later infects the body.
The Oxford COVID-19 vaccine trials
The main focus of the Phase I, II and III studies has been to assess whether the ChAdOx1 vaccine is going to work against COVID-19, that it doesn’t cause unacceptable side effects and if it induces good immune responses.
Adult participants will be randomised to receive one or two doses of either the ChAdOx1 nCoV-19 vaccine or a licensed vaccine (MenACWY) that will be used as a ‘control’ for comparison.
- Phase I: The phase I trial in healthy adult volunteers began in April 2020. More than 1,000 immunisations were given in the UK.
- Phase II: The phase II part of the study expands the age range of people the vaccine is assessed in, to include a small number of older adults and children. Researchers will be assessing the immune response to the vaccine in people of different ages, to find out if there is variation in how well the immune system responds in older people or children. The results of the Phase I/II trial were published in July 2020. Children's trials are now underway.
- Phase III: The phase III part of the study involves assessing how the vaccine works in a large number of people over the age of 18. This group will assess how well the vaccine works to prevent people from becoming infected and unwell with COVID-19. It involves multiple locations, including other countries. Initial Phase III results were published in December 2020.
For more information on the trial, including international trial locations and trial procedures, please see our COVID-19 vaccine trial information pages.
Vaccine results and production
To assess whether the vaccine works to protect from COVID-19, the statisticians in our team compare the number of infections in the control group with the number of infections in the vaccinated group. Recruitment of those who have a higher chance of being exposed to the virus has been prioritised, such as frontline healthcare workers, frontline support staff and public-facing key workers, in an effort to capture the efficacy data as quickly as possible.
The vaccine was approved for emergency use in the UK in December 2020, and has now been approved in more than 40 countries around the world.
An agreement between Oxford University and AstraZeneca means we are prepared to produce and scale up distribution of the vaccine if it is successful. We will be working closely with our partners and the British government to ensure the vaccine is made available as quickly and fairly as possible and in sufficient quantities to vaccinate the entire UK population. As part of our agreement with AstraZeneca we are ensuring that those countries who are most vulnerable to the worst effects of this global pandemic have early access to a vaccine.
This is just one of hundreds of vaccine development projects around the world; several successful vaccines offers the best possible results for humanity. Lessons learned from our work on this project are being shared with teams around the world to ensure the best chances of success.
Vaccine data and publications
Many aspects of our vaccine trials and accompanying data have been published in scientific journals.
- Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. M Voysey, S A Costa Clemens, S A Madhi, L Y Weckx, P M Folegatti, P K Aley, et al. The Lancet 2020.
- Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. M N Ramasamy, A M Minassian, K J Ewer, A L Flaxman, P M Folegatti, D R Owens, et al. The Lancet 2020.
- Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. P Folegatti, K Ewer, C Green, A Douglas, A Hill, T Lambe, S Gilbert, A Pollard et al. The Lancet 2020.
- Evaluation of the immunogenicity of prime-boost vaccination with the replication-deficient viral vectored COVID-19 vaccine candidate ChAdOx1 nCoV-19. Graham, Lambe et al. NPJ Vaccines 2020.
- ChAdOx1 nCoV-19 vaccine prevents SARS-CoV-2 pneumonia in rhesus macaques. van Doremalen, Lambe et al. Nature. 2020.
- A booster dose enhances immunogenicity of the COVID-19 vaccine candidate ChAdOx1 nCoV-19 in aged mice. Lambe, Linterman et al. Med (2020).
- Intranasal ChAdOx1 nCoV-19/AZD1222 vaccination reduces shedding of SARS-CoV-2 D614G in rhesus macaques. Lambe, Munster et al. Pre-print bioRxiv (2021).
- ChAdOx1 nCoV-19 protection against SARS-CoV-2 in rhesus macaque and ferret challenge models. Lambe, Spence et al. Pre-print ResearchSquare (2021).
- Single dose administration, and the influence of the timing of the booster dose on immunogenicity and efficacy Of ChAdOx1 nCoV-19 (AZD1222) vaccine. M Voysey, S A Costa Clemens et al. The Lancet 2021.
- Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 VOC 202012/01 (B.1.1.7). Emary, Golubchik et al. Pre-Print Preprints with THE LANCET (2021)
The University of Oxford's vaccine development work
Our vaccine work is progressing quickly. To ensure you have the latest information or to find out more about the vaccine trial, please check our latest COVID-19 research news or visit the COVID-19 vaccine trial website.




