Oxford scientists have for the first time been able to identify the origins of some severe disease-causing mutations within the testicles of normal men. This discovery will help our understanding of how certain serious genetic disorders can occur in the offspring of healthy parents, who do not themselves have the genetic defect. The research is published in the journal PNAS.
For the Oxford team, it is just the latest phase in a research programme that has been running for over 20 years. In the 1990s Professor Andrew Wilkie and colleagues were investigating a condition called Apert syndrome that affects the development of the skull and limbs. Most children with Apert syndrome are born to unaffected parents and Professor Wilkie's team showed that these cases are caused by new mutations (in a gene called FGFR2) that spontaneously arose as the father’s testes produced new sperm. Based on the prevailing knowledge of how spontaneous mutations arise, we would expect Apert syndrome to be extremely rare, but surprisingly cases occur up to 1,000 times more frequently than this.
To find out why this disease is more common than expected, Professor Anne Goriely compared sperm from fathers of children with and without Apert syndrome, and found that both groups had rare sperms with the mutation. She explained: 'The process that gives rise to Apert syndrome happens in every man, meaning any couple could have a child with Apert syndrome, regardless of the health of the parents. I also found that older men tended to produce more of these Apert mutations.' Professor Goriely also showed that normal men produce sperm with other mutations that cause lethal forms of dwarfism and other severe syndromes.
Sperm are formed when cells called spermatogonia divide into two – one of the new cells gets committed to making sperm, whereas the other stays as a spermatogonium so it can repeat the cycle. With millions of sperm and new spermatogonia being produced every day, some disease-causing mutations inevitably occur because of random errors in the complex DNA copying process. However, the team predicted that particular mutations in FGFR2 and a few other disease genes enabled the mutated spermatogonia to produce not just sperm but extra copies of themselves, reproducing and spreading faster than the surrounding normal spermatogonia. This tumour-like growth means that over time, a greater proportion of sperm being produced carry disease-causing mutations, increasing the risk of fathering a child with a serious condition. These mutations are termed ‘selfish’ because they lead to preferential growth of the mutant cells, with the associated negative effect of producing disease-causing mutations.
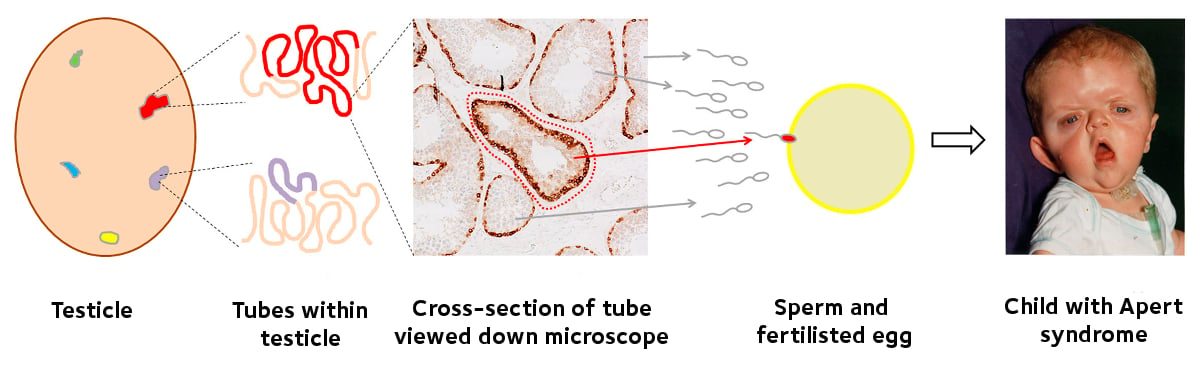 Apert syndrome: Selfish mutations spread within tubules in the testicles, producing sperm with the mutation which causes Apert syndrome. If one of these sperm fertilises the egg, then the child will have Apert syndrome.
Apert syndrome: Selfish mutations spread within tubules in the testicles, producing sperm with the mutation which causes Apert syndrome. If one of these sperm fertilises the egg, then the child will have Apert syndrome.
The current study aimed to identify these growths of mutant cells within the testicles of normal men (that were removed for surgical reasons and had been donated for research purposes). Professor Wilkie explains: 'The testis is similar to a massive tangle of spaghetti: in fact each testis contains up to 400 metres of interwoven tubes in which sperm are made. We developed techniques to pinpoint the abnormal regions of these tubes, and using a laser powered microscope, we could isolate these regions. This allowed us to perform detailed genetic analysis and we were able to identify mutations associated with severe diseases in 13 of the 14 testicles analysed.'
In essence, all men will develop these mutant growths within their testicles as they age, and with the trend for delayed parenthood, it is important that we understand the potential risks associated. Identifying these selfish growths will enable us to identify how they grow and the range of diseases that they cause in the next generation – in addition to the diseases studied, older paternal age is also associated with some cancers, autism and schizophrenia. In the clinic, non-invasive tests using blood samples from pregnant women are currently being used to screen for mutations that might be present in their babies. This work greatly adds to knowledge of which mutations are most likely to turn up – especially when the father is older.